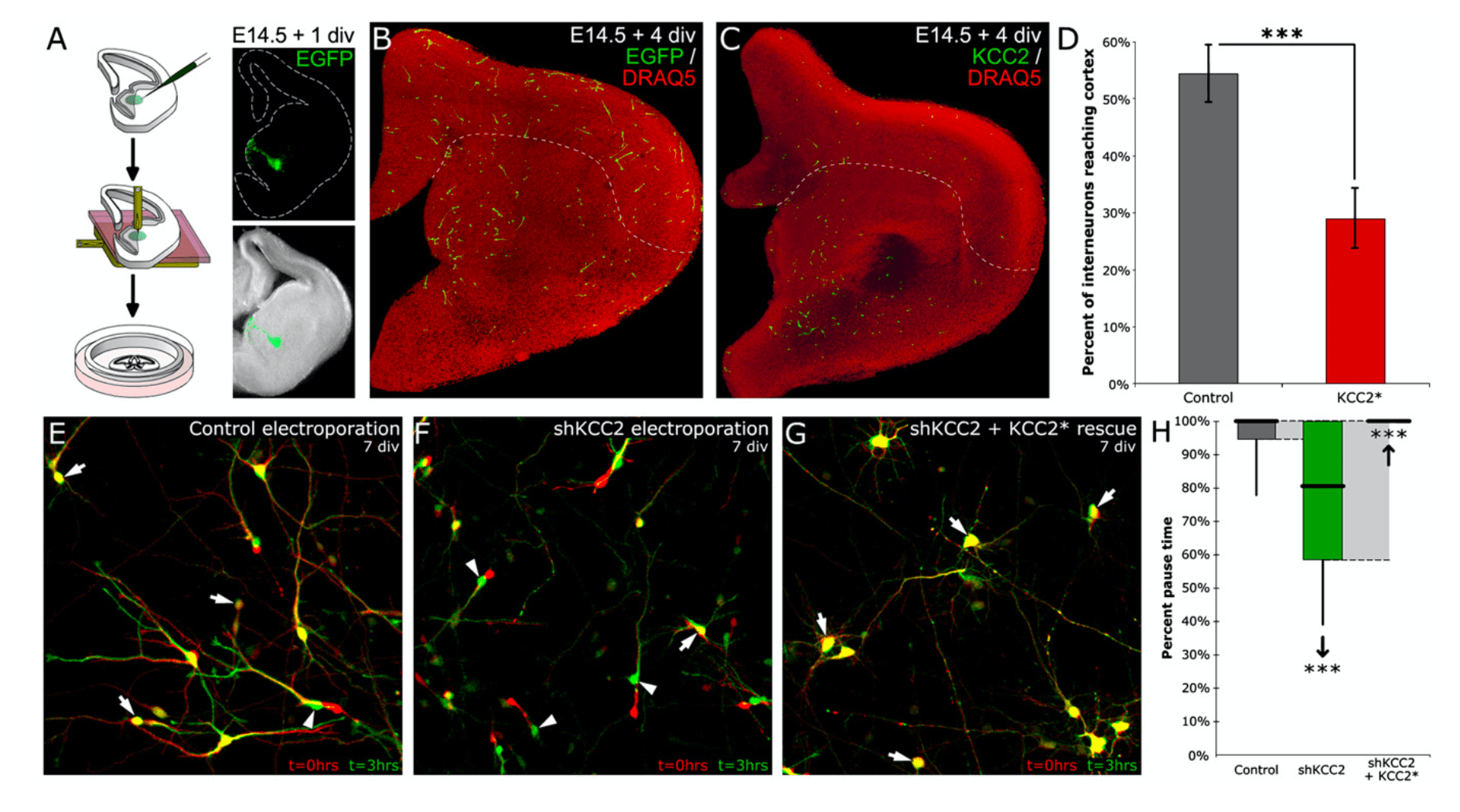
The molecular mechanisms controlling the termina- tion of cortical interneuron migration are unknown. Here, we demonstrate that, prior to synaptogenesis, migrating interneurons change their responsiveness to ambient GABA from a motogenic to a stop signal. We found that, during migration into the cortex, ambient GABA and glutamate initially stimulate the motility of interneurons through both GABAA and AMPA/NMDA receptor activation. Once in the cortex, upregulation of the potassium-chloride cotransporter KCC2 is both necessary and sufficient to reduce inter- neuron motility through its ability to reduce membrane potential upon GABAA receptor activation, which decreases the frequency of spontaneous intracellular calcium transients initiated by L-type voltage-sensi- tive calcium channel (VSCC) activation. Our results suggest a mechanism whereby migrating interneu- rons determine the relative density of surrounding interneurons and principal cells through their ability to sense the combined extracellular levels of ambient glutamate and GABA once GABAA receptor activation becomes hyperpolarizing.